Question
Buffer solutions are widely used in both chemical and biochemical systems.
Describe the composition of an acidic buffer solution.[1]
Determine the pH of a buffer solution, correct to two decimal places, showing your working, consisting of 10.0 g of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) and 10.0 g of CH\(_3\)COONa in \({\text{0.250 d}}{{\text{m}}^{\text{3}}}\) of solution. \({K_{\text{a}}}\) for \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} = 1.8 \times {10^{ – 5}}\) at 298 K.[5]
Answer/Explanation
Markscheme
(solution containing significant/equal amounts of a) weak acid and its salt / (solution containing) strong base to which excess of weak acid has been added / OWTTE;
Accept (solution containing) weak acid and conjugate base.
Do not accept descriptions with specific compounds alone (e.g. CH3COOH and CH3COONa) unless compounds are stated as weak acid and its salt.
Accept answer such as (solution containing) x mol of weak acid and \(\frac{1}{2}x\,mol\) of strong base.
\({M_{\text{r}}}{\text{(C}}{{\text{H}}_{\text{3}}}{\text{COOH)}} = 60.06\) and \({M_{\text{r}}}{\text{ C}}{{\text{H}}_3}{\text{COONa}} = 82.04\);
\({\text{[C}}{{\text{H}}_3}{\text{COOH]}} = 6.66 \times {10^{ – 1}}/0.666{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\)
\({\text{[C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ – }{\text{]}} = 4.88 \times {10^{ – 1}}/{\text{ }}0.488{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\);
\({\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{]}}/{\text{[}}{{\text{H}}^ + }{\text{]}} = (1.8 \times {10^{ – 5}} \times 6.66 \times {10^{ – 1}})/4.88 \times {10^{ – 1}} = 2.46 \times {10^{ – 5}}/0.0000246{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\);
\({\text{pH}} = \left( { – {\text{log[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{]}} = – {\text{log(2.46}} \times {\text{1}}{{\text{0}}^{ – 5}}{\text{)}} = } \right){\text{ 4.61 (2dp)}}\);
Award [5] for correct final answer of pH = 4.61 with some working shown.
Award [2 max] for pH = 4.61 without any working at all shown.
Two decimal places are required for M5.
OR
\({M_{\text{r}}}{\text{(C}}{{\text{H}}_{\text{3}}}{\text{COOH)}} = 60.06\) and \({M_{\text{r}}}{\text{ C}}{{\text{H}}_3}{\text{COONa}} = 82.04\);
\({\text{[C}}{{\text{H}}_3}{\text{COOH]}} = 6.66 \times {10^{ – 1}}/0.666{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\)
\({\text{[C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ – }{\text{]}} = 4.88 \times {10^{ – 1}}/{\text{ }}0.488{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\);
\({\text{pH}} = – {\text{log(1.8}} \times {\text{1}}{{\text{0}}^{ – 5}}{\text{)}} + {\text{log}}\frac{{{\text{[salt]}}}}{{{\text{[acid]}}}}\);
\( = \left( {4.74 + \log \frac{{0.488}}{{0.666}} = 4.74 – 0.135 = } \right){\text{ }}4.61{\text{ (2dp)}}\);
M4 can be scored even if not explicitly stated if M5 is correct based on previous values.
Award [5] for correct final answer of pH = 4.61 with some working shown.
Award [2 max] for pH = 4.61 without any working at all shown.
Two decimal places are required for M5.
Examiners report
This question was based on buffer solutions and was found to be quite challenging for candidates. In part (a), some candidates again failed to read the question, which asked for a description of an acidic buffer solution. Many did not state explicitly that a weak acid is involved (acid alone was not sufficient).
In part (b), only the best candidates scored all five marks. In addition to conceptual errors, there were also a number of transcription errors (molar mass and arithmetic errors). Candidates also were required to express their answer to two decimal places. A number of candidates used the Henderson-Hasselbalch equation, but often an incorrect equation was given.
Question
A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively.
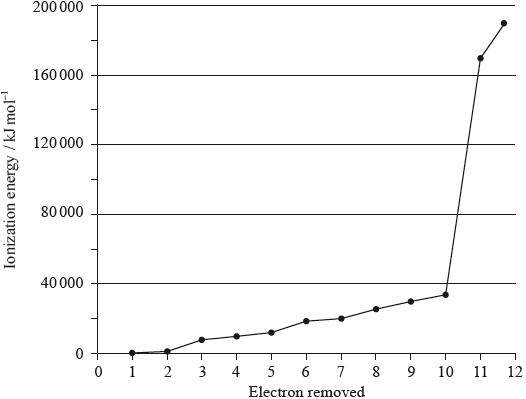
A graph of the successive ionization energies of magnesium is shown below.
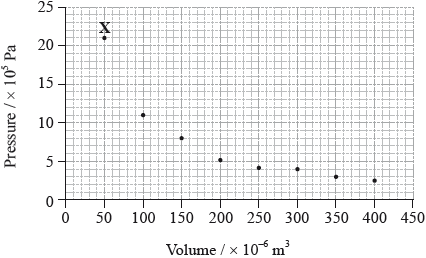
The graph below shows pressure and volume data collected for a sample of carbon dioxide gas at 330 K.
(i) Calculate the relative atomic mass of this sample of magnesium correct to two decimal places.
(ii) Predict the relative atomic radii of the three magnesium isotopes, giving your reasons.[4]
(i) Explain the increase in ionization energy values from the 3rd to the 8th electrons.
(ii) Explain the sharp increase in ionization energy values between the 10th and 11th electrons.[3]
(i) Magnesium reacts with oxygen to form an ionic compound, magnesium oxide. Describe how the ions are formed, and the structure and bonding in magnesium oxide.
(ii) Carbon reacts with oxygen to form a covalent compound, carbon dioxide. Describe what is meant by a covalent bond.
(iii) State why magnesium and oxygen form an ionic compound while carbon and oxygen form a covalent compound.[4]
(i) Predict the type of hybridization of the carbon and oxygen atoms in \({\text{C}}{{\text{O}}_{\text{2}}}\).
(ii) Sketch the orbitals of an oxygen atom in \({\text{C}}{{\text{O}}_{\text{2}}}\) on the energy level diagram provided, including the electrons that occupy each orbital.

(iii) Define the term electronegativity.
(iv) Explain why oxygen has a larger electronegativity than carbon.[7]
(i) Draw a best-fit curve for the data on the graph.
(ii) Use the data point labelled X to determine the amount, in mol, of carbon dioxide gas in the sample.[4]
(i) Most indicators are weak acids. Describe qualitatively how indicators work.
(ii) Identify a suitable indicator for a titration between a weak acid and a strong base, using Table 16 of the Data Booklet.[3]
Answer/Explanation
Markscheme
(i) \(\left( {\frac{{(77.44 \times 24) + (10.00 \times 25) + (12.56 \times 26)}}{{100}}} \right)\);
24.35;
Award [2] for correct final answer.
Two decimal places are required for M2.
Do not award any marks for 24.31 without showing method (as the value can be copied from the Data Booklet).
(ii) same atomic radii / 160 pm;
isotopes only differ by number of neutrons/size of nucleus / radius determined by electron shells and number of protons / OWTTE;
Accept neutrons do not affect distance of electrons / OWTTE.
(i) decreasing repulsion between electrons / radius decreases as electrons are removed;
Accept increasing positive charge on ion attracts electrons more strongly.
(ii) 10th electron is in second energy level/shell while 11th electron is in first energy level/shell / 10th is removing electron from electronic arrangement 2,1 while 11th ionization energy is removing electron from electronic arrangement 2;
11th electron removed is much closer to the nucleus / 11th electron removed from a (much) lower energy level/shell;
Accept opposite statement for 10th electron.
(i) magnesium (atom) gives two electrons to oxygen (atom) / oxygen (atom) takes two electrons from magnesium (atom) / magnesium (atom) loses two electrons and oxygen (atom) gains two electrons;
3-dimensional/3-D arrangement of ions / lattice of ions;
(electrostatic) attraction between oppositely charged ions/\({\text{M}}{{\text{g}}^{2 + }}\) and \({{\text{O}}^{2 – }}\);
(ii) electrostatic attraction between a pair of electrons and (positively charged) nuclei;
Accept a/two pairs of shared electrons.
(iii) difference in electronegativity is larger between Mg and O/smaller between C and O;
Accept reference to a numerical value of difference in electronegativity such as above and below 1.80.
(i) C: sp hybridization;
O: \({\text{s}}{{\text{p}}^{\text{2}}}\) hybridization;
Award [1] if the answer is sp without specifying C or O atoms.
(ii) 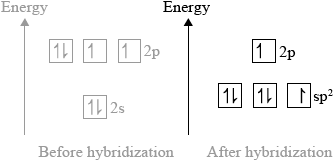
three \({\text{s}}{{\text{p}}^{\text{2}}}\) orbitals and one p-orbital at higher energy;
\({\text{s}}{{\text{p}}^{\text{2}}}\) orbitals contain: two, two and one electron and p-orbital contains one electron;
Do not allow ECF from (d)(i).
(iii) ability of atom/nucleus to attract bonding/shared pair of electrons / attraction of nucleus for bonding/shared pair of electrons / OWTTE;
(iv) (same number of shells but) increase in nuclear charge/atomic number/number of protons increases electronegativity / O has more protons than C;
Accept oxygen has a higher effective nuclear charge.
decrease in radius along the period increases electronegativity / O has smaller radius than C;
(i) smooth curve through the data;
Do not accept a curve that passes through all of the points or an answer that joins the points using lines.
(ii) \(p = 21 \times {10^5}/2.1 \times {10^6}{\text{ (Pa)}}/2.1 \times {10^3}{\text{ (kPa)}}\) and
\(V = 50 \times {10^{ – 6}}/5.0 \times {10^{ – 5}}{\text{ }}({{\text{m}}^3})/5.0 \times {10^{ – 2}}{\text{ }}({\text{d}}{{\text{m}}^3})\);
\(\left( {n = \frac{{pV}}{{RT}}} \right)\frac{{2.1 \times {{10}^6} \times 5.0 \times {{10}^{ – 5}}}}{{8.31 \times 330}}\);
\(n = 0.038{\text{ (mol)}}\);
Award [3] for correct final answer.
For M3 apply ECF for correct computation of the equation the student has written, unless more than one mistake is made prior this point.
(i) equilibrium between HIn and \({\text{I}}{{\text{n}}^ – }/{\text{HIn}} \rightleftharpoons {\text{I}}{{\text{n}}^ – } + {{\text{H}}^ + }\);
the colours of HIn and \({\text{I}}{{\text{n}}^ – }\) are different;
if added to acid, the equilibrium shifts to the left and the colour of HIn is seen / OWTTE;
if added to base/alkali, the equilibrium shifts to the right and the colour of \({\text{I}}{{\text{n}}^ – }\) is seen / OWTTE;
(ii) phenolphthalein;
Accept phenol red.
Examiners report
(i) Most candidates were able to calculate the relative atomic mass to the correct number of decimal places.
(ii) Only strong candidates were able to predict the same radius for the isotopes and gave correct reasoning. However, the majority of candidates predicted that a larger number of neutrons resulted is a smaller radius, reflecting a poor understanding of atomic structure.
(i) Very few candidates were able to explain the increase in successive ionization energies for electrons removed from the same sub-shell. Many candidates gave incorrect reasoning.
(ii) The increase between the 10th and 11th ionization energies of magnesium was explained correctly by about half of the candidates. Few candidates scored the first mark by identifying the correct shells or sub-shells the electrons are removed from.
(i) Well answered by many candidates. A few candidates were confusing ionic with covalent bonding, and some referred to a linear MgO molecule in an ionic lattice.
(ii) Few candidates were able to describe the covalent bond precisely. Those who didn’t score usually didn’t make any reference to pairs of electrons.
(iii) Many candidates obtained this mark with satisfactory arguments. It was disappointing to see the abundance of answers based on “is a metal with a non-metal” or “both are non-metals”.
(i) A few candidates identified sp hybridization based on a linear structure. Only the strongest candidates were able to give the correct hybridization for oxygen as well.
(ii) This was the most challenging question on the paper. It was rare to see a correct answer. It seems candidates did not have a good understanding of hybridization.
(iii) Less than half the candidates were able to define electronegativity precisely. Many candidates did not relate it to the pair of electrons in a covalent bond, and simply talked about attracting electrons, which was not sufficient for the mark.
(iv) Many candidates gained the first mark by stating that oxygen has more protons than carbon. But very few candidates identified the second factor, which is the smaller radius of oxygen.
(i) More than half of the candidates drew a smooth curve that was central to the data points. Errors included straight lines, curves joining all data points, or a curve that was not central to the points.
(ii) A very well answered question. Some candidates converted the units of p and V incorrectly and others did not read the scales of the graph correctly.
(i) Many candidates could explain the behaviour of indicators, but there were also some poor answers that did not acknowledge the importance of equilibrium in the action of an indicator.
(ii) Most candidates suggested a suitable indicator.
