Question
Consider these standard electrode potentials.
\[\begin{array}{*{20}{l}} {{\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ – } \rightleftharpoons {\text{Mg(s)}}}&{{E^\Theta } = – 2.36{\text{ V}}} \\ {{\text{Z}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ – } \rightleftharpoons {\text{Zn(s)}}}&{{E^\Theta } = – 0.76{\text{ V}}} \end{array}\]
What is the cell potential for the voltaic cell produced when the two half-cells are connected?
A. –1.60 V
B. +1.60 V
C. –3.12 V
D. +3.12 V
▶️Answer/Explanation
B
The zinc half-cell will undergo reduction because its standard reduction potential is higher. The Mg half-cell will undergo oxidation.
Cell potential = (-0.76) + 2.36 = +1.60 V
Question
What is the cell potential, in V, for the reaction that occurs when the following two half-cells are connected?
\[\begin{array}{*{20}{l}} {{\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ – } \rightleftharpoons {\text{Fe(s)}}}&{{E^\Theta } = – 0.44{\text{ V}}} \\ {{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }({\text{aq)}} + 14{{\text{H}}^ + }({\text{aq)}} + 6{{\text{e}}^ – } \rightleftharpoons 2{\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}}&{{E^\Theta } = + 1.33{\text{ V}}} \end{array}\]
A. +0.01
B. +0.89
C. +1.77
D. +2.65
▶️Answer/Explanation
C
The Cr half-cell will undergo reduction because its standard reduction potential is higher. The Fe half-cell will undergo oxidation.
Cell potential = +1.33 – (-0.44) = +1.77 V.
Question
Which are necessary conditions for the standard hydrogen electrode to have an \({E^\Theta }\) of exactly zero?
I. Temperature = 298 K
II. \({\text{[}}{{\text{H}}^ + }{\text{]}} = 1{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\)
III. \({\text{[}}{{\text{H}}_2}{\text{]}} = 1{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
A
In standard hydrogen electrode 1M HCl is taken which means \({\text{[}}{{\text{H}}^ + }{\text{]}} = 1{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) and hydrogen gas is taken at 1atm pressure and temperature is maintained at 25℃ or 298K. In these conditions its standard reduction potential and standard oxidation potential is always zero. This is the reason it can be used as a reference electrode.
Question
A voltaic cell is made by connecting two half-cells represented by the half-equations below.
\[\begin{array}{*{20}{l}} {{\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ – } \to {\text{Mn(s)}}}&{{E^\Theta } = – 1.19{\text{ V}}} \\ {{\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ – } \to {\text{Pb(s)}}}&{{E^\Theta } = – 0.13{\text{ V}}} \end{array}\]
Which statement is correct about this voltaic cell?
A. Mn is oxidized and the voltage of the cell is 1.06 V.
B. Pb is oxidized and the voltage of the cell is 1.06 V.
C. Mn is oxidized and the voltage of the cell is 1.32 V.
D. Pb is oxidized and the voltage of the cell is 1.32 V.
▶️Answer/Explanation
A
The Pb half-cell will undergo reduction because its standard reduction potential is higher. The Mn half-cell will undergo oxidation.
Cell potential = (-0.13) – (-1.19) = -0.13 +1.19 = +1.06V.
Question
For the electrolysis of aqueous copper(II) sulfate using graphite electrodes, which of the following statements is correct?
A. Cu and \({{\text{O}}_{\text{2}}}\) are produced in a mol ratio of 1:1
B. \({{\text{H}}_{\text{2}}}\) and \({{\text{O}}_{\text{2}}}\) are produced in a mol ratio of 1:1
C. Cu and \({{\text{O}}_{\text{2}}}\) are produced in a mol ratio of 2:1
D. \({{\text{H}}_{\text{2}}}\) and \({{\text{O}}_{\text{2}}}\) are produced in a mol ratio of 2:1
▶️Answer/Explanation
C
You have a mixture of Cu2+,SO42-, and H2O.
Copper(II) undergoes reduction to Cu,
Cu2+(aq)+2e–→Cu(s);+0.34 V
and as SO42- is already in its highest oxidation state, water undergoes oxidation to form oxygen gas.
2H2O(l)→O2(g)+4H+(aq)+4e– ; -1.23 V
Overall reaction is :
2Cu2+(aq)+2H2O(l)→2Cu(s)+O2(g)+4H+(aq) ;-0.89 V
Thus, if you electrolyze a solution of CuSO4 using graphite electrodes, you will see metallic carbon being deposited at the cathode and bubbles of oxygen released at the anode.
So, Cu and \({{\text{O}}_{\text{2}}}\) are produced in a mol ratio of 2:1.
Question
What are the major products of electrolyzing concentrated aqueous potassium iodide, KI(aq)?
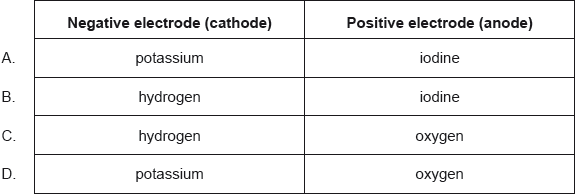
▶️Answer/Explanation
B
In the electrolysis of an aqueous solution of potassium iodide, I– ions are oxidized at the anode preferentially to water molecules. Violet color at anode is due to iodine.
At cathode, water molecules are reduced and hydrogen gas is evolved.
