Question
Consider the following reversible reaction.
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{2CrO}}_4^{2 – }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}}\]
What will happen to the position of equilibrium and the value of \({K_{\text{c}}}\) when more \({{\text{H}}^ + }\) ions are added at constant temperature?
▶️Answer/Explanation
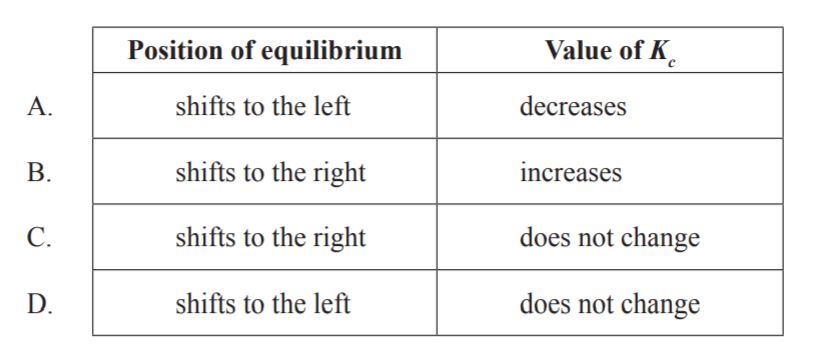
D
As temperature is kept constant, Kc does not change.
According to Le Chatelier’s principle: When additional reactant is added, the equilibrium shifts to reduce this stress: it makes more product. When additional product is added, the equilibrium shifts to reactants to reduce the stress.
So, If H+ is added, there is now more product, so the reaction will shift toward reactants to reduce the added H+ . Hence, reaction shifts to the left.
Question
Which statement is correct for the equilibrium \({{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}_2}{\text{O(g)}}\) in a closed system at 100 °C?
A. All the \({{\text{H}}_{\text{2}}}{\text{O(l)}}\) molecules have been converted to \({{\text{H}}_{\text{2}}}{\text{O(g)}}\).
B. The rate of the forward reaction is greater than the rate of the reverse reaction.
C. The rate of the forward reaction is less than the rate of the reverse reaction.
D. The pressure remains constant.
▶️Answer/Explanation
D
The container was closed and at constant temperature hence equilibrium has been established. The rate of forward reaction is equal to the rate of backward reaction. Pressure now remains constant.
Question
What is the effect of an increase of temperature on the yield and the equilibrium constant for the following reaction?
\[\begin{array}{*{20}{l}} {{\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} + {\text{CO(g)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}}&{\Delta {H^\Theta } = – 128{\text{ kJ}}} \end{array}\]
▶️Answer/Explanation
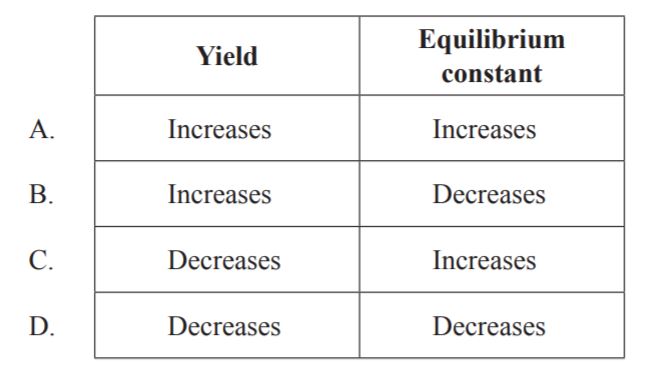
D
Because
Question
Which of the following will shift the position of equilibrium to the right in the Haber process?
\({{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\) \(\Delta {H^\Theta } = {\text{-92.6 kJ}}\)
I. Decreasing the concentration of \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\)
II. Decreasing the temperature
III. Increasing the pressure
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
D
In the Haber process, the forwards reaction is exothermic.
Exothermic: energy + reactants → products + energy.
Decreasing temperature means decreasing energy i.e. decreasing products, hence, equilibrium will shift towards products.
If NH3 is removed, there is now less product, so the reaction will shift toward products to replace the product removed.
According to Le Chatelier’s principle, if pressure is increased, then the equilibrium shifts to the side with the fewer number of moles of gas. This particular reaction shows a total of 4 mol of gas as reactants and 2 mol of gas as products, so the reaction shifts toward the products side.
Question
Which statements explain why a catalyst is used in the Contact process (shown below)?
\[{\text{S}}{{\text{O}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{S}}{{\text{O}}_3}{\text{(g)}}\]
I. A catalyst creates a new reaction pathway of lower activation energy.
II. A catalyst moves the position of equilibrium towards the product.
III. A catalyst allows the same rate to be achieved at a lower temperature.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
B
A catalyst creates a new reaction pathway of lower activation energy.
A catalyst allows the same rate to be achieved at a lower temperature.
Adding a catalyst does not affect the relative rates of the two reactions, it cannot affect the position of equilibrium.
