Question
Which statement is always correct for a chemical reaction at equilibrium?
A. The rate of the forward reaction equals the rate of the reverse reaction.
B. The amounts of reactants and products are equal.
C. The concentration of the reactants and products are constantly changing.
D. The forward reaction occurs to a greater extent than the reverse reaction.
▶️Answer/Explanation
A
Chemical equilibrium is the state of a system in which the rate of the forward reaction is equal to the rate of the reverse reaction.
Question
An increase in temperature increases the amount of chlorine present in the following equilibrium.
\[{\text{PC}}{{\text{l}}_5}({\text{s)}} \rightleftharpoons {\text{PC}}{{\text{l}}_3}({\text{l)}} + {\text{C}}{{\text{l}}_2}({\text{g)}}\]
What is the best explanation for this?
A. The higher temperature increases the rate of the forward reaction only.
B. The higher temperature increases the rate of the reverse reaction only.
C. The higher temperature increases the rate of both reactions but the forward reaction is affected more than the reverse.
D. The higher temperature increases the rate of both reactions but the reverse reaction is affected more than the forward.
▶️Answer/Explanation
C
With increase in temperature, the rate of the reaction and the rate constant increases. Which means, rate of both forward and backward reaction increases. Since, an increase in temperature increases the amount of chlorine present in the equilibrium, which is possible when the forward rate increases by more than the backward rate and the forward reaction is affected more than the reverse.
Question
What will happen when at a constant temperature, more iodide ions, \({{\text{I}}^ – }\), are added to the equilibrium below?
\[{{\text{I}}_{\text{2}}}{\text{(s)}} + {{\text{I}}^ – }{\text{(aq)}} \rightleftharpoons {\text{I}}_3^ – {\text{(aq)}}\]
A. The amount of solid iodine decreases and the equilibrium constant increases.
B. The amount of solid iodine decreases and the equilibrium constant remains unchanged.
C. The amount of solid iodine increases and the equilibrium constant decreases.
D. The amount of solid iodine increases and the equilibrium constant remains unchanged.
▶️Answer/Explanation
B
\(k=\frac{[I_3]}{[I_2][I^-]}\)
At constant temperature, equilibrium constant remains unchanged.
When the concentration of a component in an equilibrium is increased, the rate of the reaction in which that substance is a reactant is increased; more of the product of that reaction is formed.
As concentration of iodide ions is increased, The equation that consumes iodide ions is the forward reaction,hence rate of forward reaction increases and hence the amount of solid iodine decreases
Question
What is the equilibrium constant expression, \({K_{\text{c}}}\), for the following reaction?
\[{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{O}}_{\text{2}}}{\text{(g)}}\]
A. \({K_{\text{c}}} = \frac{{[{\text{N}}{{\text{O}}_2}]}}{{[{{\text{N}}_2}{{\text{O}}_4}]}}\)
B. \({K_{\text{c}}} = \frac{{{{[{\text{N}}{{\text{O}}_2}]}^2}}}{{[{{\text{N}}_2}{{\text{O}}_4}]}}\)
C. \({K_{\text{c}}} = \frac{{[{\text{N}}{{\text{O}}_2}]}}{{{{[{{\text{N}}_2}{{\text{O}}_4}]}^2}}}\)
D. \({K_{\text{c}}} = [{\text{N}}{{\text{O}}_2}]{[{{\text{N}}_2}{{\text{O}}_4}]^2}\)
▶️Answer/Explanation
B
\[{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{O}}_{\text{2}}}{\text{(g)}}\]
Equilibrium constant, \({K_{\text{c}}} = \frac{{{{[{\text{N}}{{\text{O}}_2}]}^2}}}{{[{{\text{N}}_2}{{\text{O}}_4}]}}\)
Question
Consider the endothermic reaction below.
\[{\text{5CO(g)}} + {{\text{I}}_2}{{\text{O}}_5}{\text{(g)}} \rightleftharpoons {\text{5C}}{{\text{O}}_2}{\text{(g)}} + {{\text{I}}_2}{\text{(g)}}\]
According to Le Chatelier’s principle, which change would result in an increase in the amount of \({\text{C}}{{\text{O}}_2}\)?
A. Increasing the temperature
B. Decreasing the temperature
C. Increasing the pressure
D. Decreasing the pressure
▶️Answer/Explanation
A
When the temperature of an equilibrium mixture is increased, the rate of both reactions increases , but the rate of the endothermic reaction (the reaction that absorbs the added energy) is increased more. Here, given that forward reaction is endothermic, hence, increase in temperature would result in an increase in the amount of \({\text{C}}{{\text{O}}_2}\).
Question
What happens to the position of equilibrium and the value of \({K_{\text{c}}}\) in the following reaction when the temperature is decreased?
\({{\text{N}}_2}{{\text{O}}_4}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{O}}_2}{\text{(g)}}\) \(\Delta {H^\Theta } = + 57.2{\text{ kJ}}\)
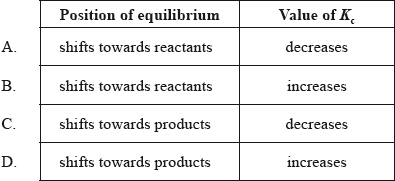
▶️Answer/Explanation
A
When the temperature of an equilibrium mixture is decreased, the rate of both reactions decreases , but the rate of the endothermic reaction (the reaction that absorbs the added energy) is decreased more.
Here, forward reaction has ΔH > 0; i.e. it is endothermic. It means rate of forward reaction (endothermic) decreases and rate of backward reaction increases here.
Thus, position of equilibrium shifts towards reactants and value of \({K_{\text{c}}}\) decreases.
