Question
The molar mass of a compound is approximately \({\text{56 g}}\,{\text{mo}}{{\text{l}}^{ – 1}}\). Which formula is possible for this compound?
A. \({\text{NaN}}{{\text{O}}_{\text{3}}}\)
B. AgOH
C. MgO
D. KOH
▶️Answer/Explanation
D
\({\text{NaN}}{{\text{O}}_{\text{3}}}\) : 23+14+16×3 = 85
AgOH : 108+16+1 = 125
MgO : 24+16 = 40
KOH : 39+16+1 = 56
Answer : D.
Question
Which compound has the empirical formula with the largest mass?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
C. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
D. \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\)
▶️Answer/Explanation
D
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\) : 12×2 + 1×6 = 30
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) : 12×2 + 1×4 = 28
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\) : 12×2 + 1×2 = 26
\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\) : 12×3 + 1×6 = 42
Correct answer is D.
Question
Which non-metal forms an oxide XO2 with a relative molecular mass of 60?
A. C
B. N
C. Si
D. S
▶️Answer/Explanation
C
Given, molecular mass = 60.
X + 2×16 = 60
X = 28
Here, Si has atomic mass 28.
Question
4.00 mol of a hydrocarbon with an empirical formula of \({\text{C}}{{\text{H}}_{\text{2}}}\) has a mass of 280 g. What is the molecular formula of this compound?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
B. \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\)
C. \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\)
D. \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}\)
▶️Answer/Explanation
D
Mass of 4 moles of hydrocarbon = 280 g
Mass of 1 mole of Hydrocarbon = 70 g
Out of these, mass of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}\) = 12×5+1×10 = 70.
Hence, correct answer is D.
Question
What is the coefficient of \({\rm{F}}{{\rm{e}}_3}{{\rm{O}}_4}\) when the following equation is balanced using the lowest whole numbers?
__ \({\text{Al(s)}} + \) __ \({\text{F}}{{\text{e}}_3}{{\text{O}}_4}({\text{s)}} \to \) __ \({\text{A}}{{\text{l}}_2}{{\text{O}}_3}({\text{s)}} + \) __ \({\text{Fe(s)}}\)
A. 2
B. 3
C. 4
D. 5
▶️Answer/Explanation
B
Since the equation is to be balanced in whole numbers,
To balance O atoms, Place the coefficient of 3 in front of \({\text{F}}{{\text{e}}_3}{{\text{O}}_4}({\text{s)}} \) and the coefficient of 4 in front of \({\text{A}}{{\text{l}}_2}{{\text{O}}_3}({\text{s)}}\)
Now, to balance Fe, place 9 in front of Fe(s)
To balance Al, place 8 in front of Al(s).
Hence, coefficient of \({\rm{F}}{{\rm{e}}_3}{{\rm{O}}_4}\) is 3.
Question
For which compound is the empirical formula the same as the molecular formula?
Ar(H)=1; Ar(C)=12; Ar(O)=16
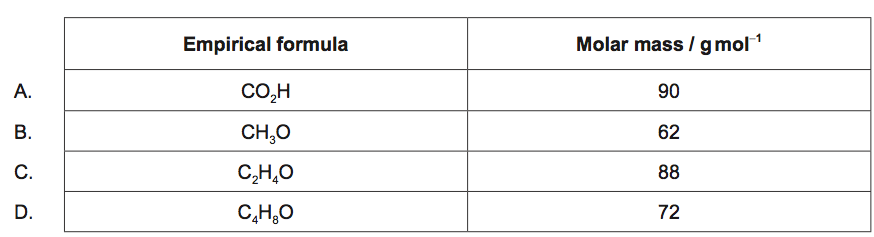
▶️Answer/Explanation
D
For empirical formula to be same as the molecular formula,
mass of empirical formula must be equal to the molar mass of the compound.
Out of these, mass of C4H8O is 4×12+8×1+16 = 72 g which is also its molar mass.
