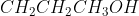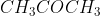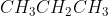Question
Which series is in order of increasing boiling point?
A |
|
|
|
B |
|
|
|
C |
|
|
|
D |
|
|
|
▶️Answer/Explanation
Ans: B
Alcohols have H bonding, hence highest boiling point.
Alkanes are non polar, hence lowest boiling point.
Ketones are polar molecules, and there are two lone pairs on the oxygen atom of the carbonyl group. They will have more boiling pointy than alkanes but lesser than alcohols.
Question
What is the IUPAC name for \({\text{HCOOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)?
A. Butanoic acid
B. Butanal
C. Methyl propanoate
D. Propyl methanoate
▶️Answer/Explanation
D
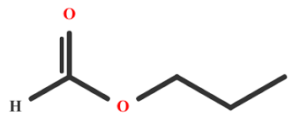
Question
What is the IUPAC name of the compound \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)?
A. Ethyl ethanoate
B. Propyl ethanoate
C. Ethyl propanoate
D. Pentyl propanoate
▶️Answer/Explanation
C
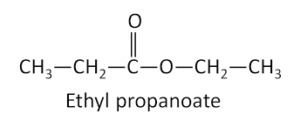
Question
Which compound is an amide?
A. CH3COOCH3
B. CH3CONH2
C. CH3NH2
D. CH2(NH2)COOH
▶️Answer/Explanation
B
An amide is a functional group that consists of a carbonyl group and a nitrogen atom. B is an amide.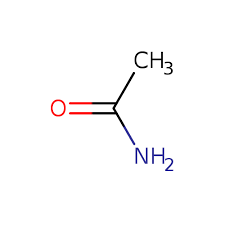
Question
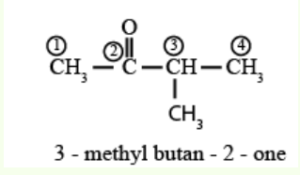
What is the name of \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CHCOC}}{{\text{H}}_{\text{3}}}\) applying IUPAC rules?
A. 3,3-dimethylpropan-2-one
B. 3-methylbutan-2-one
C. 2-methylbutan-3-one
D. 3-methylbutanal
▶️Answer/Explanation
B