Question
Which is in the same homologous series as 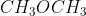 ?
?
A 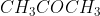
B 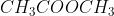
C 
D 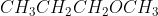
▶️Answer/Explanation
Ans: D
CH3OCH3 is dimethyl ether. Here, an oxygen atom is linked to two methyl groups.
CH3CH3CH2OCH3 is also an ether. Here, an oxygen atom is linked to one propyl group and other methyl group.
Question
Which three compounds can be considered to be a homologous series?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{CHO, C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}{\text{, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH, (C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}{\text{COH}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{, (C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
▶️Answer/Explanation
A
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\) is an homologous series as all these compounds belong to R-OH group i.e. Alcohols. The alcohol functional group involves an oxygen atom that is bonded to one hydrogen atom and one carbon atom.
Question
What is the IUPAC name for \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)C}}{{\text{H}}_{\text{3}}}\)?
A. 1,1-dimethylpropane
B. 2-ethylpropane
C. 2-methylbutane
D. 3-methylbutane
▶️Answer/Explanation
C
It has longest Carbon chain having 4 carbon atoms hence it is butane and it has one methyl group at second carbon in the chain, hence it is 2-methyl butane.
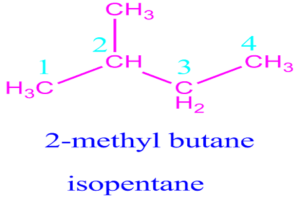
Question
Which is a tertiary halogenoalkane?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)Cl}}\)
C. \({\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}{\text{Br}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHClC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
▶️Answer/Explanation
C
In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different.
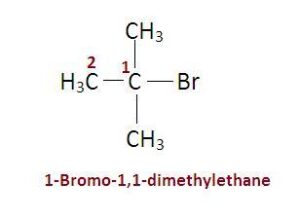
Here, 3 methyl groups are attached directly to the carbon atom holding Br.
Question
What is the IUPAC name of the following compound?
A. 2-methylbutane
B. Ethylpropane
C. 3-methylbutane
D. Pentane
▶️Answer/Explanation
A
It has longest Carbon chain having 4 carbon atoms hence it is butane and it has one methyl group at second carbon in the chain, hence it is 2-methyl butane.
Question
How many structural isomers exist with the formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{5}}}{\text{C}}{{\text{l}}_{\text{3}}}\)?
A. 3
B. 4
C. 5
D. 6
▶️Answer/Explanation
C
It has 5 structural isomers:
1,1,1-trichloropropane,
1,2,3-trichloropropane,
1,2,2-trichloropropane,
1,1,2-trichloropropane and 1,1,3-trichloropropane.
