Question
The table below shows the number of protons, neutrons and electrons present in five species.
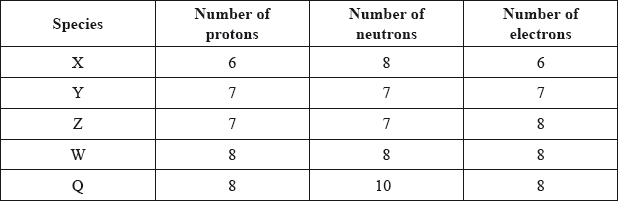
Which two species are isotopes of the same element?
A. X and W
B. Y and Z
C. Z and W
D. W and Q
▶️Answer/Explanation
D
Isotopes have same atomic number but different mass number. Hence, they have equal number of protons and electrons and different mass number.
Here, W and Q have equal number of protons i.e. 8 each and equal number of electrons i.e. 8 each. Hence, they are isotopes.
As mass number is equal to protons + neutrons in an atom.
Mass number of W = 8+8 = 16. and Mass number of Q = 8+10 = 18, which is different.
Question
Which quantities are the same for all atoms of chlorine?
I. Number of protons
II. Number of neutrons
III. Number of electrons
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
B
All atoms of chlorine include its isotopes. Chlorine has two stable isotopes: Cl-35 and Cl-37 .
Isotopes have same atomic number but different mass number. Hence, they have equal number of protons and electrons and different mass number.
Hence, all atoms of chlorine will have the same number of protons and the same number of electrons.
Question
Consider the relative abundance of the isotopes of element X.
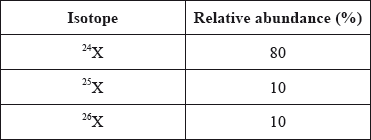
What is the relative atomic mass of X?
A. 24
B. 25
C. Between 24 and 25
D. Between 25 and 26
▶️Answer/Explanation
C
\(Relative Atomic mass = \frac{Isotropic mass \times Percentage Abundance }{100}\)
\(Relative Atomic mass = \frac{24\times 80 + 25\times 10 + 26\times 10}{100}\)
Relative Atomic mass = 24.3 = Between 24 and 25.
Question
What are the numbers of neutrons and electrons in the iodine ion, \(^{{\text{125}}}{{\text{I}}^ + }\)?
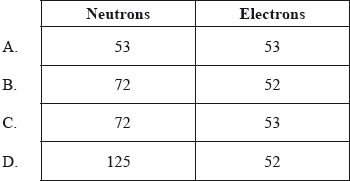
▶️Answer/Explanation
B
Here, 125 is the mass number. As we know, 53 is the atomic number of iodine and +1 is the given charge on the atom.
Since atomic number is equal to the number of electrons on neutral atom, neutral I atom would have had 53 electrons.
But here we also have a positive charge of 1. So, total number of electrons = 53-1 = 52.
Number of Protons = Atomic number = 53.
Atomic mass = Number of Protons + number of Neutrons
Number of Neutrons = 125 – 53 = 72.
Question
Which representation would be correct for a species, Z, which has 31 protons, 40 neutrons and 28 electrons?
A. \({}_{31}^{71}{{\rm{Z}}^{3 + }}\)
B. \({}_{31}^{71}{{\rm{Z}}^{3 – }}\)
C. \({}_{40}^{71}{{\rm{Z}}^{3 + }}\)
D. \({}_{28}^{71}{{\rm{Z}}^{3 + }}\)
▶️Answer/Explanation
A
Number of Protons = Atomic number = 31.
Number of electrons = Atomic number – Positive charge
Positive charge = 31-28 = 3.
Atomic mass = Number of Protons + number of Neutrons
Atomic mass = 31+40 = 71.
Hence, answer is A.
