Question
The diagram shows the first ionisation energies of consecutive elements in the same period
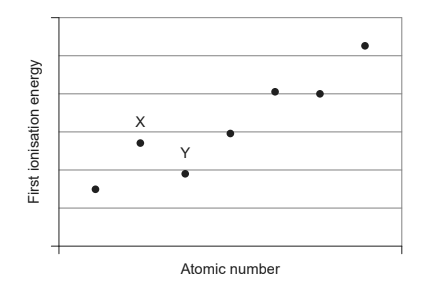
Which factor explains why element X has a higher first ionisation energy than element Y?
A Element Y loses an electron from a different sub-level.
B Element Y has a smaller atomic radius.
C Element X has a full octet.
D Element Y has a greater nuclear charge.
▶️Answer/Explanation
Ans: A
The outer electron in element X could be in an s sub-shell. However, the outer electron in Y could be in a p sub-shell, so it is higher in energy than the outer electron in X. This means that less energy is needed to remove it. Hence, Y has less first ionisation energy than X.
Question
Which species possesses only two unpaired electrons?
A. Zn
B. Mg
C. \({\text{T}}{{\text{i}}^{2 + }}\)
D. \({\text{F}}{{\text{e}}^{2 + }}\)
▶️Answer/Explanation
C
Option A : The electron configuration of Zinc is 1s22s22p63s23p64s23d10. All of the electron orbital in the first three energy levels are filled.
The 4s sub level is also filled and the 3d sub shell is filled. There are no unpaired electrons in Zinc.
Option B : The ground state of Mg is 1s22s22p63s2 so all electrons are paired. No electrons are unpaired here.
Option C : Electronic configuration of titanium is [Ar] 4s2 3d2. In the case of Ti, there are four valence electrons, two in the 4s and two in the 3d. When the +2 ion is formed the 4s electrons are lost, leaving the two 3d electrons which are in different orbitals, and therefore, unpaired. So the bottom line is that Ti2+ has two unpaired electrons.
Option D : Fe2+=[Ar]3d6 Four unpaired electron are present in the Fe2+ ion.
Question
What is the order of increasing energy of the orbitals within a single energy level?
A. \({\text{d}} < {\text{s}} < {\text{f}} < {\text{p}}\)
B. \({\text{s}} < {\text{p}} < {\text{d}} < {\text{f}}\)
C. \({\text{p}} < {\text{s}} < {\text{f}} < {\text{d}}\)
D. \({\text{f}} < {\text{d}} < {\text{p}} < {\text{s}}\)
▶️Answer/Explanation
B
Increasing energy of the orbitals within a single energy level is \({\text{s}} < {\text{p}} < {\text{d}} < {\text{f}}\) .
Question
What is the electron configuration of the \({\text{C}}{{\text{r}}^{2 + }}\) ion?
A. \({\text{[Ar]3}}{{\text{d}}^{\text{5}}}{\text{4}}{{\text{s}}^{\text{1}}}\)
B. \({\text{[Ar]3}}{{\text{d}}^{\text{3}}}{\text{4}}{{\text{s}}^{\text{1}}}\)
C. \({\text{[Ar]3}}{{\text{d}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{1}}}\)
D. \({\text{[Ar]3}}{{\text{d}}^{\text{4}}}{\text{4}}{{\text{s}}^{\text{0}}}\)
▶️Answer/Explanation
D
Correct Electron Configuration for Chromium (Cr) is 1s2 2s2 2p6 3s2 3p6 3d5 4s1
For the Cr2+ ion we remove one electron from 4s1 and one from the 3d5 leaving us with: 1s22s22p63s23p63d4
Which is \({\text{[Ar]3}}{{\text{d}}^{\text{4}}}{\text{4}}{{\text{s}}^{\text{0}}}\)
Question
What is the electron configuration of vanadium?
A. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{2}}}{\text{4}}{{\text{s}}^{\text{3}}}\)
B. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{3}}}{\text{4}}{{\text{s}}^{\text{2}}}\)
C. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{4}}}{\text{4}}{{\text{s}}^{\text{1}}}\)
D. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{5}}}\)
▶️Answer/Explanation
B
Atomic number of V = 23
Electron configuration of vanadium is : \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{3}}}{\text{4}}{{\text{s}}^{\text{2}}}\)
Question
Which shows the sub-levels in order of increasing energy in the fourth energy level of an atom?
A. \({\text{f}} < {\text{d}} < {\text{p}} < {\text{s}}\)
B. \({\text{p}} < {\text{d}} < {\text{f}} < {\text{s}}\)
C. \({\text{d}} < {\text{f}} < {\text{p}} < {\text{s}}\)
D. \({\text{s}} < {\text{p}} < {\text{d}} < {\text{f}}\)
▶️Answer/Explanation
D
Increasing energy of the orbitals within a single energy level (here, fourth energy level) is \({\text{s}} < {\text{p}} < {\text{d}} < {\text{f}}\) .
