Question
How are emission spectra formed?
A. Photons are absorbed when promoted electrons return to a lower energy level.
B. Photons are absorbed when electrons are promoted to a higher energy level.
C. Photons are emitted when electrons are promoted to a higher energy level.
D. Photons are emitted when promoted electrons return to a lower energy level.
▶️Answer/Explanation
Ans: D
Emission spectra is formed when the electrons in an atom return from a higher (excited) state to lower energy levels, thereby emitting energy in the form of photons.
Question
Which is correct for the following regions of the electromagnetic spectrum?
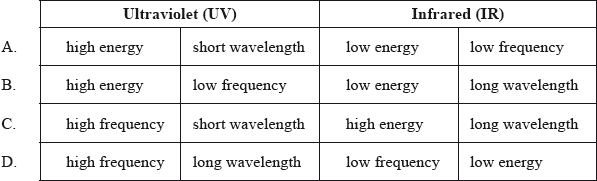
▶️Answer/Explanation
A
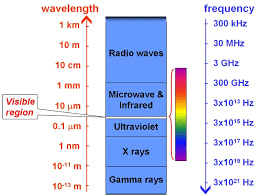
Here, Ultraviolet (UV) region has high frequency, high energy and short wavelength.
Infrared (IR) region has low frequency, low energy and long wavelength.
Question
The diagram represents the emission spectrum of hydrogen. Groups of arrows are labelled W, X and Y.
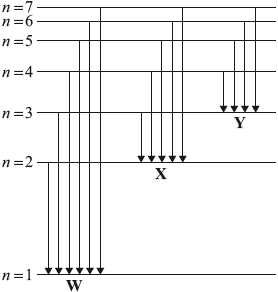
Which statement is correct?
A. The arrows represent the transition of electrons to different energy levels when heat is supplied.
B. The arrows of W represent emission in the UV region.
C. The smallest arrow of X represents a violet line in the emission spectrum.
D. The arrows of Y represent emission of electromagnetic waves with higher energy than those represented by X and W.
▶️Answer/Explanation
B
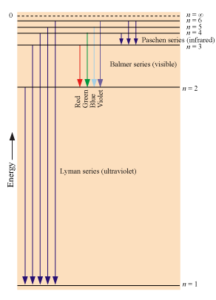
Spectral series is obtained when an atom emits energy while making transition from higher energy level to lower energy level. Different lines in the spectral series corresponds to different amounts of energy released.
Here, W is Lyman series (Ultraviolet).
X is Balmer series (Visible) with Smallest arrow being Red and Largest being Violet.
Y is Paschen series (Infrared).
Arrows of Y have Infrared series which has lesser energy than X (Visible) and W (Ultariolet) series.
Question
Ultraviolet radiation has a shorter wavelength than infrared radiation. How does the frequency and energy of ultraviolet radiation compare with infrared radiation?
▶️Answer/Explanation
A
\(E=h\nu \)
\(E=\frac{hc}{\lambda }\)
Since wavelength is inversely proportional to energy and frequency both, hence, Ultraviolet radiation will have higher frequency and higher energy than infrared radiation.
Question
Which is correct for the line emission spectrum for hydrogen?
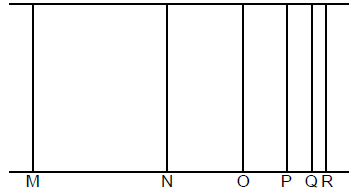
A. Line M has a higher energy than line N.
B. Line N has a lower frequency than line M.
C. Line M has a longer wavelength than line N.
D. Lines converge at lower energy.
▶️Answer/Explanation
C
The given lines represent emission spectrum for hydrogen.
There are infinitely many spectral lines, but they become very dense as they approach n equal to infinity.
Wavelength decreases from M to R. Lines converge at higher energy.
Line M corresponds to n=2 and line N corresponds to n=3. Hence, line M has longer wavelength, higher frequency and higher energy than line N.
