Question
What is the electron domain geometry of Si in Si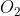 ?
?
A bent
B linear
C square planar
D tetrahedral
▶️Answer/Explanation
Ans: D
SiO2 is a covalent, 3-D network solid. Each Si atom is covalently bonded in a tetrahedral manner to four oxygen atoms. Each oxygen atom in turn covalently bonded to another silicon atom. Each corner is shared with another tetrahedron.
Question
Which molecule contains a dative covalent (coordinate) bond?
A. HCN
B. \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\)
C. \({\text{C}}{{\text{O}}_{\text{2}}}\)
D. CO
▶️Answer/Explanation
D
In CO, there is a triple bond between C and O. Out of these three bonds, two are formed by sharing of electrons (covalent bond) between C and O. And one bond is a coordination bond, in which the Oxygen donated its lone pair of electrons to the Carbon atom.
Question
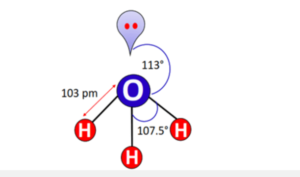
What is the bond angle in the \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) ion?
A. 104°
B. 107°
C. 109°
D. 120°
▶️Answer/Explanation
B
The ideal bond angle in a tetrahedral molecule is approx. equal to 109.5°. But it is due to a lone pair of electrons on the central O-atom in [H3O]+ that lone pair-bond pair repulsions push the left and right O-H bonds away from the lone pair. The internal H-O-H bond angle decreases to 107.5 ° while the external bond angle increases up to an experimentally determined value of 113°, as shown in the figure below.
Question
Allotropes of fullerene contains how many carbon atom?
A. 35
B. 60
C. 50
D. 45
▶️Answer/Explanation
B
Fullerene, one of the allotropes of carbon has 60 carbon atoms.
Question
Which molecule has an octahedral shape?
A. SF6
B. PCl5
C. XeF4
D. BF3
▶️Answer/Explanation
A
SF6 molecule is made up of 6 equally spaced sp3d2 hybrid orbitals arranged at 90o angles. The shape of the orbitals is octahedral. Since there is an atom at the end of each orbital, the shape of the molecule is also octahedral.
Question
The Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) is given below.
![]()
What is the shape of the \({\text{S}}{{\text{O}}_{\text{2}}}\) molecule?
A. Bent (V-shaped)
B. Linear
C. T-shaped
D. Triangular planar
▶️Answer/Explanation
A
SO2 molecular geometry is considered to V-shaped or bent. Alternatively, the electron geometry of sulphur dioxide is in the shape of a trigonal planar. The three pairs of bonding electrons lie at an angle of 119o. Two double pairs are bonded together and there is one lone pair as well which further gives it a bent shape.
Question
Which species contain dative covalent bonds?
I. CO
II. \({\text{N}}{{\text{H}}_{\text{3}}}\)
III. \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
B
In CO, there is a triple bond between C and O. Out of these three bonds, two are formed by sharing of electrons (covalent bond) between C and O. And one bond is a coordinate (dative) bond, in which the Oxygen donated its lone pair of electrons to the Carbon atom.
\({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) ion has two O-H covalent bonds and and one O-H covalent coordinate(dative) bond.
