Question
Which molecule is polar?
A. \({\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\)
B. \({\text{BC}}{{\text{l}}_{\text{3}}}\)
C. \({\text{C}}{{\text{l}}_{\text{2}}}\)
D. \({\text{CC}}{{\text{l}}_{\text{4}}}\)
▶️Answer/Explanation
A
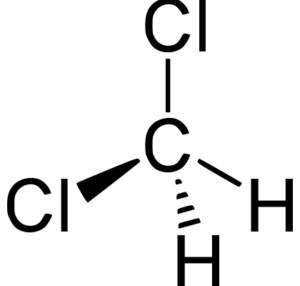
CH2Cl2 is a polar molecule due to its tetrahedral geometrical shape and difference between the electronegativity of Carbon, Hydrogen and Chlorine atoms. This develops a dipole moment across C-Cl and C-H bonds and the entire molecule results in a net 1.67 D dipole moment.
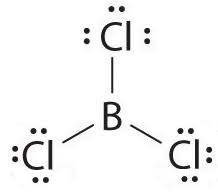
Boron Trichloride or BCl3 is a nonpolar compound because of its symmetrical structure ie; Trigonal Planar. The B-Cl bond itself is polar because of the difference in electronegativity of Boron(2.04) and Chlorine(3.16) atoms and all three B-Cl bonds lie at 120 degrees to each other. As a result, the dipole moment of each B-Cl bond is canceled out by each other making the net dipole moment zero and the entire molecule nonpolar in nature.
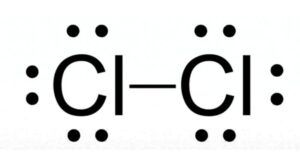
Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. As a result, both atoms have equal charge distribution on them, and the molecule results in zero dipole moment that makes the chlorine molecule nonpolar.
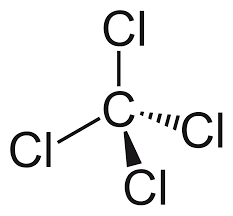
CCl4 (Carbon tetrachloride) is nonpolar in nature. Although the four bonds C-Cl are polar because of the difference in electronegativity of Chlorine(3.16) and Carbon(2.55), CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure (tetrahedral) of the CCl4 molecule.
Question
Which row correctly describes the bonding type and melting point of carbon and carbon dioxide?
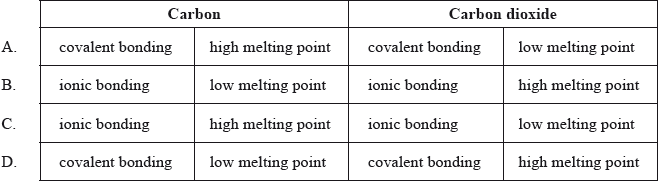
▶️Answer/Explanation
A
Carbon dioxide is a simple covalent compound, consisting of discrete molecules. The molecules are held together by weak intermolecular forces of attraction. Very little energy is needed to break these weak bonds, hence carbon dioxide has low melting point and boiling point.
Carbon mainly exists in its allotropic forms. Diamond, graphite, fullerene, and carbon nanotubes are prominent allotropes of carbon. In diamond and graphite structures, carbon has giant covalent molecular structure where each carbon atom is covalently bonded to four other carbon atoms. These covalent bonds are very strong and a lot of energy is required to separate these atoms. Hence, carbon has very high melting point.
Question
\({{\text{C}}_{{\text{60}}}}\) fullerene consists of a simple molecular structure. Silicon dioxide, \({\text{Si}}{{\text{O}}_{\text{2}}}\), can be described as a giant covalent (macromolecular) structure. Which statements are correct?
I. Each carbon atom in \({{\text{C}}_{{\text{60}}}}\) fullerene is bonded in a sphere of 60 carbon atoms, consisting of pentagons and hexagons.
II. Each O–Si–O bond angle in \({\text{Si}}{{\text{O}}_{\text{2}}}\) is 180°.
III. \({\text{Si}}{{\text{O}}_{\text{2}}}\) is insoluble in water.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
B
Buckminsterfullerene (C60) is a spherical carbon allotrope where 60 atoms are assembled in pentagons and hexagons, in a geometry similar to a soccer ball.
The fullerene, C60, consists of fused five and six-membered carbon rings. Each six membered rings is surrounded, alternately, by hexagons and pentagons of carbons; each pentagon is fused to five hexagons.
Silicon dioxide (SiO2) is a covalent molecule with four oxygen atoms connected to each silicon atom and two oxygen atoms bonded to each silicon atom in a tetrahedral structure. Silicon dioxide has a large structure with an eight-membered ring produced by the arrangement of alternate oxygen and silicon atoms. Each O–Si–O bond angle in \({\text{Si}}{{\text{O}}_{\text{2}}}\) is 109.5° the tetrahedral structure.
Question
What describes the structure of silicon and silicon dioxide?
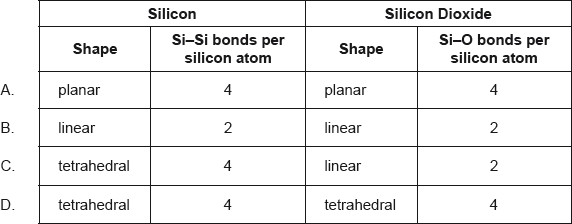
▶️Answer/Explanation
D
In a silicon crystal, each silicon shares four electrons with four neighboring silicon atoms in tetrahedral shape. A silicon atom has 14 electrons around the nucleus, and of these, there are 4 valence electrons on the outermost orbital. Every atom sees the full complement of 18 electrons. This situation is very much like the covalent bonding of carbon, which lies just above silicon.
The silicon atom is tetrahedrally coordinated in the bulk of silicates, with four oxygen atoms around a core Si atom. As a result, SiO2 produces three-dimensional network solids in which each silicon atom is covalently connected to four oxygen atoms in a tetrahedral fashion.
Question
What are the predicted electron domain geometries around the carbon and both nitrogen atoms in urea, (NH2)2CO, applying VSEPR theory?
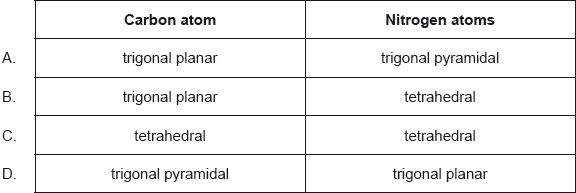
▶️Answer/Explanation
B
The Lewis structure of urea is: 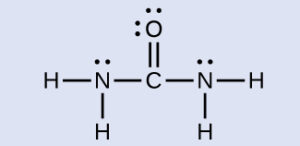
The nitrogen atoms are surrounded by four regions of electron density, one lone pair and 3 single bond pairs, which arrange themselves in a tetrahedral electron-pair geometry. The hybridization in a tetrahedral arrangement is sp3 . This is the hybridization of the nitrogen atoms in urea.
The carbon atom is surrounded by three regions of electron density, one double bond pair and two single bond pairs, positioned in a trigonal planar arrangement. The hybridization in a trigonal planar electron pair geometry is sp2, which is the hybridization of the carbon atom in urea.
