Question
Which compound has the greatest volatility under the same conditions?
A S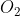
B Si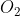
C Sn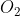
D SrO
▶️Answer/Explanation
Ans: A
Volatility refers to the ease in which it changes state. SiO2 is a giant covalent structure, so it has high melting point as covalent bonds need to be broken for a change of state & SrO is an ionic compound so it will also have a relatively high melting point. SO2 and SnO2 are both covalent structures, so they will only have LDF’s. LDFs increase with electron number, so SnO2, the larger molecule, will have stronger intermolecular forces than SO2. Hence, SO2 will be most volatile, as it has the easiest forces to overcome.
Question
What is the correct order if the compounds are arranged in order of increasing boiling point?
A. \({\text{C}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{Si}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{Si}}{{\text{H}}_{\text{4}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{Si}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{4}}}\)
D. \({\text{C}}{{\text{H}}_{\text{4}}} < {\text{Si}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
▶️Answer/Explanation
D
Due to the presence of intermolecular hydrogen bonding in alcohols large energy is required to break these bonds and thus alcohols have high boiling points. So, CH3OH will have highest boiling point. CH3Cl is polar it has second highest boiling point. SiH4 and CH4 are non polar. They have weak Van der Waals forces They will have the lowest boiling points.. SiH4has larger size so it has more boiling point than CH4.
Question
Which substance can form intermolecular hydrogen bonds in the liquid state?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OC}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
▶️Answer/Explanation
B
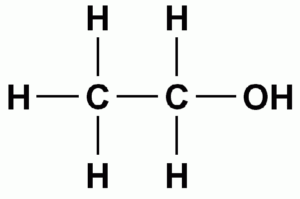
Out of these, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\) can form hydrogen bond. For the formation of hydrogen bond, at least one hydrogen atom must be bonded to one of the three most electronegative atoms, i.e, O,N and F.
Question
Which combination of properties is correct?
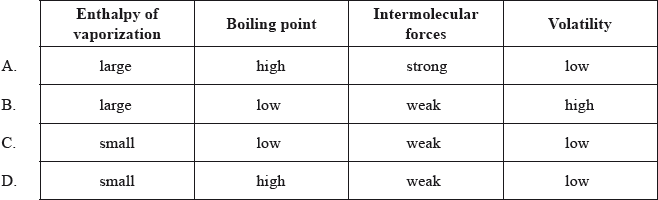
▶️Answer/Explanation
A
As enthalpy of vaporization increases, it means intermolecular forces are strong and boiling point is high and volatility is low.
Question
Which correctly lists butane \({\text{(}}{M_{\text{r}}} = {\text{58)}}\), propanone \({\text{(}}{M_{\text{r}}} = {\text{58)}}\), propan-1-ol \({\text{(}}{M_{\text{r}}} = {\text{60)}}\) and propan-2-ol
\({\text{(}}{M_{\text{r}}} = {\text{60)}}\) in order of increasing boiling point?
A. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}} < {{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\)
C. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
D. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\)
▶️Answer/Explanation
A
2∘ -Alcohols have lower boiling points than 1∘ -alcohols due to a corresponding decrease in the extent of H-bonding because of steric hindrance. Therefore Propan -1-ol has higher boiling point than Propan -2-ol.
