Question
According to the Brønsted-Lowry theory, how does each species act in the equilibrium below?
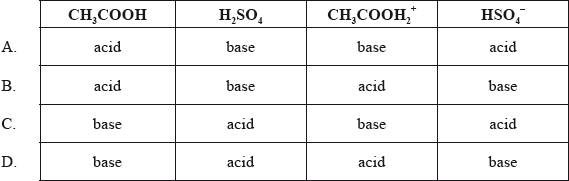
\[{\text{C}}{{\text{H}}_3}{\text{COOH}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{COOH}}_2^ + + {\text{HSO}}_4^ – \]
▶️Answer/Explanation
D
According to Bronsted-Lowry theory, acid is a substance which donates an H+ ion or a proton and forms its conjugate base and the base is a substance which accepts an H+ ion or a proton and forms its conjugate acid.
Here, CH3COOH accepts H+ ion, it is a base with CH3COOH2+ as its conjugate acid. H2SO4 donates an H+ ion and it is an acid with HS04– as its conjugate base.
Question
Which of the following is an example of a Lewis acid–base reaction, but not a Brønsted–Lowry acid–base reaction?
A. \({\text{2CrO}}_4^{2 – }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 – }{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\)
B. \({\text{Co(}}{{\text{H}}_2}{\text{O)}}_6^{2 + }{\text{(aq)}} + {\text{4HCl(aq)}} \to {\text{CoCl}}_4^{2 – }{\text{(aq)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}}\)
C. \({\text{N}}{{\text{H}}_3}{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}} \to {\text{NH}}_4^ + {\text{(aq)}}\)
D. \({\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ – }{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} + {\text{O}}{{\text{H}}^ – }{\text{(aq)}}\)
▶️Answer/Explanation
B
Lewis Acids are the chemical species which have empty orbitals and are able to accept electron pairs from Lewis bases.
Here, \({\text{Co(}}{{\text{H}}_2}{\text{O)}}_6^{2 + }{\text{(aq)}}\) acts as Lewis acid and accepts electron pair from Cl– which acts as Lewis base.
Here, no donation or acceptance of proton takes place, hence it is not a Brønsted–Lowry acid–base reaction.
Question
What are the conjugate acid–base pairs in the following reaction?
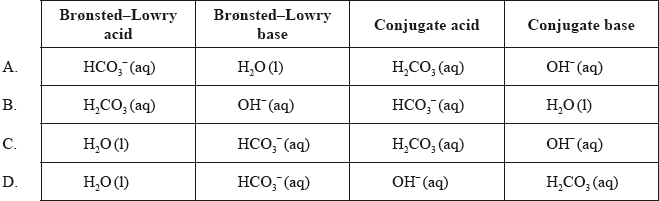
\[{\text{HCO}}_3^ – {\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{O}}{{\text{H}}^ – }{\text{(aq)}} + {{\text{H}}_2}{\text{C}}{{\text{O}}_3}{\text{(aq)}}\]
▶️Answer/Explanation
C
\({\text{HCO}}_3^ – {\text{(aq)}}\) accepts H+. It acts as Bronsted-Lowry base. Its conjugate acid is H2CO3.
\({{\text{H}}_2}{\text{O(l)}}\) donates H+. It acts as Bronsted-Lowry acid. Its conjugate base is OH–.
Question
What is the conjugate base of phenol, \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{OH}}\)?
A. \({{\text{C}}_6}{\text{H}}_4^ – \)–OH
B. \({{\text{C}}_6}{{\text{H}}_5}\)–\(\mathop {\text{O}}\limits^ + {{\text{H}}_2}\)
C. \({{\text{C}}_6}{{\text{H}}_5}\)–\({{\text{O}}^ – }\)
D. \({{\text{C}}_6}{\text{H}}_6^ + \)–OH
▶️Answer/Explanation
C
A Bronsted-Lowry base is a substance which accepts a proton or H+ ion. Conjugate base of Phenol is \({{\text{C}}_6}{{\text{H}}_5}\)–\(\mathop {\text{O}}\limits^ + {{\text{H}}_2}\).
Question
Which species produced by the successive dissociations of phosphoric acid, H3PO4, are amphiprotic?
A. HPO42− and PO43−
B. H2PO4− and HPO42−
C. H2PO4− and PO43−
D. HPO42− only
▶️Answer/Explanation
B
Amphiprotic species are H2PO4− and HPO42− as they can behave as acid as well as a base.
