Question
Which salts will dissolve in water to give solutions with a pH above 7?
I. \({\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\)
II. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COONa}}\)
III. \({\text{N}}{{\text{a}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
A
Carbonic acid is a weak acid and sodium hydroxide is a strong base and Na2CO3 is formed by their reaction. Hence, Na2CO3 is a basic salt, pH>7.
Because it was created by a strong base (NaOH) and a weak acid(CH3COOH), CH3COONa is a basic salt, pH>7.
Sodium sulphate (Na2SO4) is formed by a reaction between sodium hydroxide and sulphuric acid which are strong base and acid, respectively. Thus, it is a neutral salt with a pH of 7.
Question
Which group of three compounds contains only weak acids and bases?
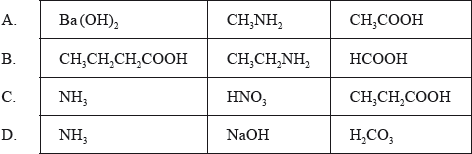
▶️Answer/Explanation
B
Carboxylic acids are weak acids and amines are weak bases.
Question
The table below shows data for the \({{K_{\text{a}}}}\) and \({{\text{p}}{K_{\text{b}}}}\) values for some acids and bases at 298 K.

Which two formulas represent the weakest acid and the weakest base in the table?
A. HClO and \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
B. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\) and \({\text{N}}{{\text{H}}_{\text{3}}}\)
C. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\) and \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
D. HClO and \({\text{N}}{{\text{H}}_{\text{3}}}\)
▶️Answer/Explanation
A
The one with lesser Ka is weak acid. So, HClO is weaker acid.
The one with more pKb is weaker base, hence C6H5NH2 is weaker base here.
Question
II. The strong acid reacts with a metal oxide but the weak acid does not.
III. The strong acid has greater conductivity than the weak acid.
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
B
Weak acid dissociates less.
Both weak and strong acid will react with basic metal oxides.
The strong acid has greater conductivity than the weak acid because it dissociates more into ions.
