Question
Alloys containing at least $60 \%$ copper reduce the presence of bacteria on their surface. The percentage of copper in brass, an alloy of copper and zinc, can be determined by UV-vis spectrometry.
A sample of brass is dissolved in concentrated nitric acid and then made up to $250.0 \mathrm{~cm}^3$ with water before analysis.
$$
\begin{aligned}
& \mathrm{Cu}(\mathrm{s})+4 \mathrm{HNO}_3(\mathrm{aq}) \rightarrow \mathrm{Cu}\left(\mathrm{NO}_3\right)_2(\mathrm{aq})+2 \mathrm{NO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{ll}) \\
& 3 \mathrm{Zn}(\mathrm{s})+8 \mathrm{HNO}_3(\mathrm{aq}) \rightarrow 3 \mathrm{Zn}\left(\mathrm{NO}_3\right)_2(\mathrm{aq})+2 \mathrm{NO}(\mathrm{g})+4 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \\
&
\end{aligned}
$$
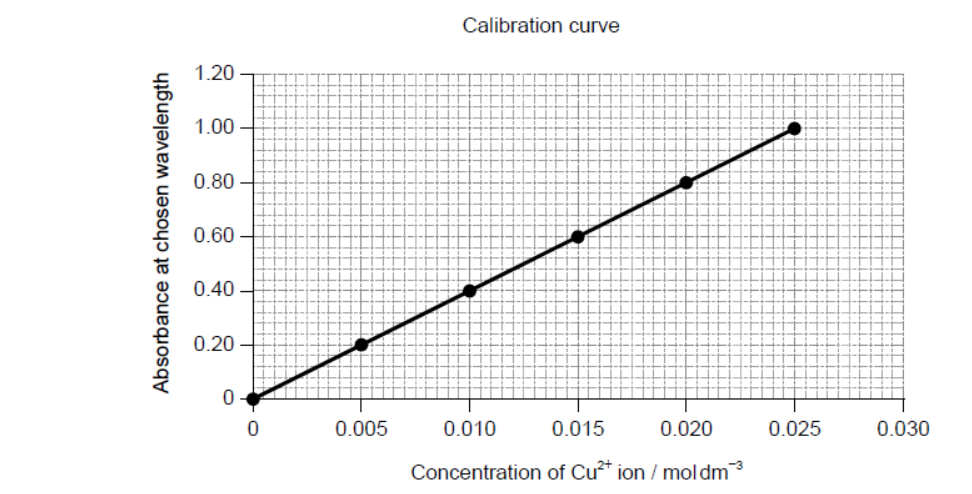
The concentration of copper(II) ions in the resulting solution is then determined from a calibration curve, which is plotted by measuring the light absorbance of standard solutions.
You may find the following chart and diagram helpful.
a. Outline why the initial reaction should be carried out under a fume hood.
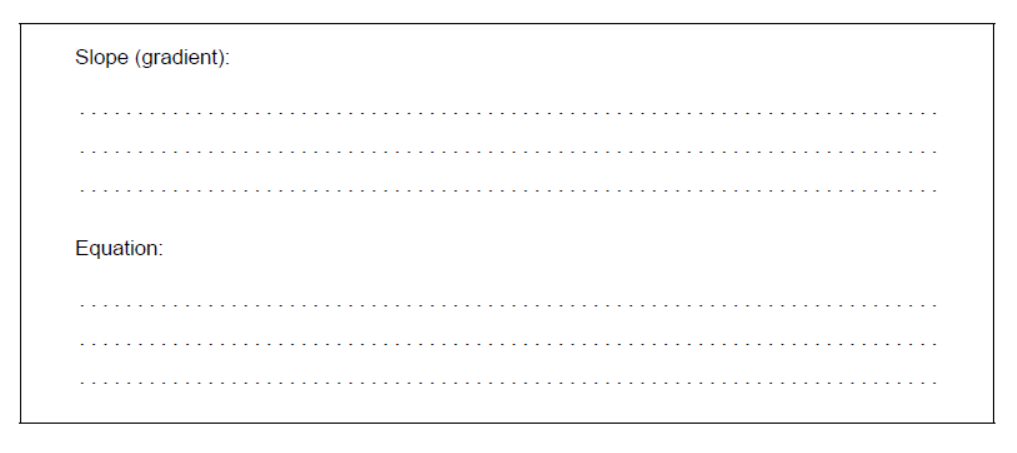
b. Deduce the equation for the relationship between absorbance and concentration.
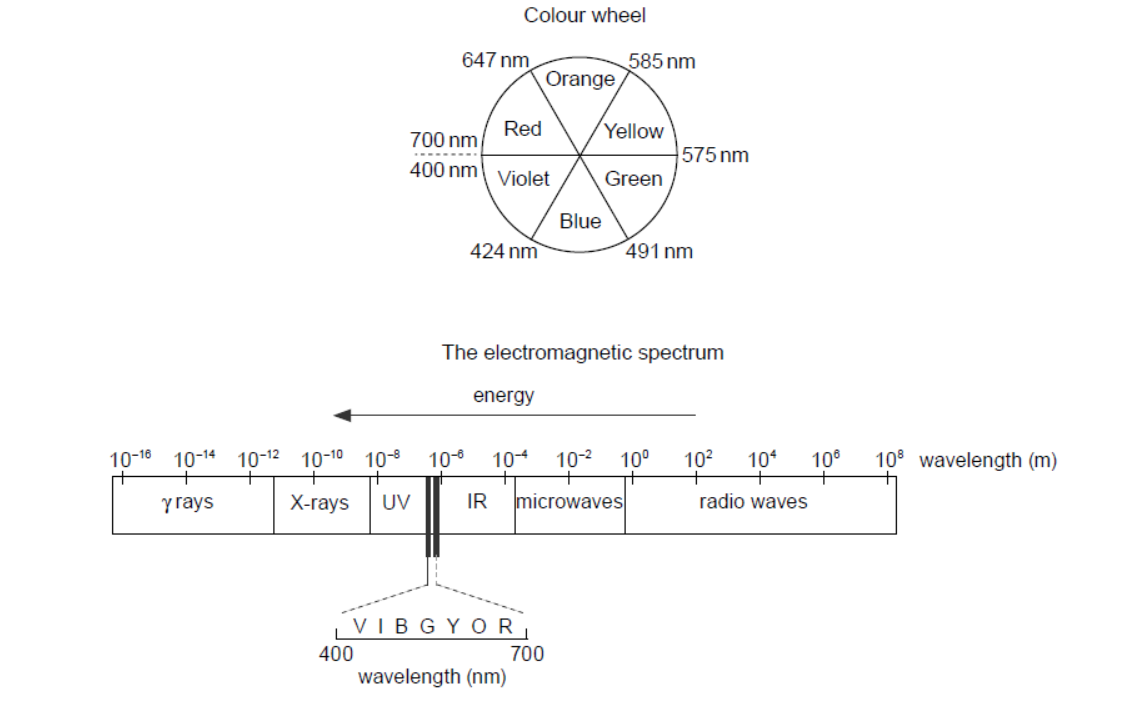
c. Copper(II) ion solutions are blue. Suggest, giving your reason, a suitable wavelength of light for the analysis.
d. Outline how a solution of $0.0100 \mathrm{~mol} \mathrm{dm}^{-3}$ is obtained from a standard $1.000 \mathrm{~mol} \mathrm{dm}^{-3}$ copper(II) sulfate solution, including two essential pieces of glassware you would need.
e.i. The original piece of brass weighed $0.200 \mathrm{~g}$. The absorbance was 0.32 .
Calculate, showing your working, the percentage of copper by mass in the brass.
e.ii. Deduce the appropriate number of significant figures for your answer in (e)(i).
f.i. Comment on the suitability of using brass of this composition for door handles in hospitals.
If you did not obtain an answer to (e)(i), use $70 \%$ but this is not the correct answer.
f.ii. Suggest another property of brass that makes it suitable for door handles.
g. Titration is another method for analysing the solution obtained from adding brass to nitric acid.
Copper(II) ions are reduced to copper(I) iodide by the addition of potassium iodide solution, releasing iodine that can be titrated with sodium thiosulfate solution, $\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3(\mathrm{aq})$. Copper(I) iodide is a white solid.
$$
\begin{gathered}
4 \mathrm{I}^{-}(\mathrm{aq})+2 \mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cul}(\mathrm{s})+\mathrm{I}_2(\mathrm{aq}) \\
\mathrm{I}_2(\mathrm{aq})+2 \mathrm{~S}_2 \mathrm{O}_3{ }^{2-}(\mathrm{aq}) \rightarrow 2 \mathrm{I}^{-}(\mathrm{aq})+\mathrm{S}_4 \mathrm{O}_6{ }^{2-}(\mathrm{aq})
\end{gathered}
$$
Suggest why the end point of the titration is difficult to determine, even with the addition of starch to turn the remaining free iodine black.
▶️Answer/Explanation
Markscheme
a. $\mathrm{NO}_2 / \mathrm{NO} / \mathrm{NO}_{\mathrm{x}} / \mathrm{HNO}_3 /$ gas is poisonous/toxic/irritant
Accept formula or name.
Accept ” $\mathrm{HNO}_3$ is corrosive” OR “poisonous/toxic gases produced”.
Accept “reaction is harmful/hazardous”.
b. Slope (gradient):
40
Equation:
absorbance $=40 \times$ concentration
OR
$y=40 x$
Accept any correct relationship for slope such as $\frac{1.00}{0.025}$.
Award [2] if equation in M2 is correct.
c. orange is opposite blue «in the colour wheel»
OR
the complementary colour «blue» is seen/transmitted
$585-647$ «nm would be absorbed»
Accept any value or range within 550-680 «nm» for M2.
d. dilute $1.00 \mathrm{~cm}^3$ «of the standard solution with water» to $100 \mathrm{~cm}^3$
OR
dilute sample of standard solution «with water» 100 times
«graduated/volumetric» pipette/pipet
volumetric flask
Accept any 1: 100 ratio for $M 1$.
Accept “mix $1 \mathrm{~cm}^3$ of the standard solution with $99 \mathrm{~cm}^3$ of water” for M1.
Do not accept “add $100 \mathrm{~cm}^3$ of water to $1.00 \mathrm{~cm}^3$ of standard solution” for M1.
Accept “burette/buret” for M2.
Accept “graduated/measuring flask” for M3 but not “graduated/measuring cylinder” or “conical/Erlenmeyer flask”.
e.i. concentration of copper $=0.0080 \ll \mathrm{mol} \mathrm{dm}^{-3} » \sim$
mass of copper in $250.0 \mathrm{~cm}^3=« 0.0080 \mathrm{~mol} \mathrm{dm}^{-3} \times 0.2500 \mathrm{dm}^3 \times 63.55 \mathrm{~g} \mathrm{~mol}^{-1}=» 0.127$ «”
OR
mass of brass in $1 \mathrm{dm}^3=« 4 \times 0.200 \mathrm{~g}=» 0.800 \mathrm{~g} \mathrm{AND}$ [Cu2+] $=« 0.0080 \mathrm{~mol} \mathrm{dm}^{-3} \times 63.55 \mathrm{~g} \mathrm{~mol}^{-1}=» 0.5084 \mathrm{~g} \mathrm{dm}^{-3}$
$« \%$ copper in this sample of brass $=\frac{0.127}{0.200} \times 100=» 64$ «\%»
OR
$« \%$ copper in this sample of brass $=\frac{0.5084}{0.800} \times 100=» 64 \ll \%$
Accept any value in range $0.0075-0.0085$ «mol $\mathrm{dm}^3$ » for M1.
Accept annotation on graph for M1.
Award [3] for correct final answer.
Accept “65 «\%»”.
e.ii.two
Do not apply ECF from 1(e)(i).
f.i. «since it is greater than $60 \%$ » it will reduce the presence of bacteria «on door handles»
f.ii. resistant to corrosion/oxidation/rusting
OR
low friction surface «so ideal for connected moving components»
Accept “hard/durable”, “«high tensile» strength”, “unreactive”, “malleable” or any reference to the appearance/colour of brass (eg “gold-like”, “looks nice” etc.).
Do not accept irrelevant properties, such as “high melting/boiling point”, “non-magnetic”, “good heat/electrical conductor”, “low volatility”, etc. Do not accept “ductile”.
g. precipitate/copper(I) iodide/Cul makes colour change difficult to see
OR
release of $\mathrm{I}_2$ /iodine from starch- $\mathrm{I}_2$ complex is slow so titration must be done slowly
Question
The exhaust gases of automobiles contribute significantly to air pollution in cities.
Outline how the pollutant gases nitrogen(II) oxide, NO, nitrogen(IV) oxide, \({\text{N}}{{\text{O}}_2}\) and carbon monoxide, CO, are formed as a result of the action of the internal combustion engine.
NO:
\({\text{N}}{{\text{O}}_2}\):
CO:
▶️Answer/Explanation
Markscheme
NO:
\({{\text{N}}_{\text{2}}}\) and \({{\text{O}}_{\text{2}}}\) react in the engine / \({{\text{N}}_{\text{2}}} + {{\text{O}}_{\text{2}}} \to {\text{2NO}}\);
No mark for the high temperature without reference to the action between N2 and O2.
\(N{O_2}\):
NO oxidizes/reacts in the air to \({\text{N}}{{\text{O}}_{\text{2}}}\) / x;
\({\text{2NO}} + {{\text{O}}_{\text{2}}} \to {\text{2N}}{{\text{O}}_{\text{2}}}\);
CO:
incomplete combustion;
Accept balanced chemical equation for C5–C12 hydrocarbons.
Do not accept C1–C4.
Examiners report
Many candidates identified correctly how the three gases are formed, though some candidates named the reaction of \({{\text{N}}_{\text{2}}}\) and \({{\text{O}}_{\text{2}}}\) as the source of \({\text{N}}{{\text{O}}_{\text{2}}}\) and for incomplete combustion equations with methane and carbon were given.
Question
The two major acids that cause acid rain originate from different sources.
State an equation that shows why rain water is naturally acidic.
Outline the process responsible for the production of each acid and state an equation to show its formation.
Acid rain has caused damage to limestone buildings and marble statues. State an equation to represent the reaction of acid rain with limestone or marble.
▶️Answer/Explanation
Markscheme
\({\text{C}}{{\text{O}}_2} + {{\text{H}}_{\text{2}}}{\text{O}} \rightleftharpoons {{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}/{\text{C}}{{\text{O}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}} \rightleftharpoons {{\text{H}}^ + } + {\text{HCO}}_3^ – \);
Do not penalize absence of reversible sign.
Do not accept CO2 + H2O \( \to \) 2H+ + CO32–.
Acid 1:
\({\text{(HN}}{{\text{O}}_{\text{2}}}{\text{/HN}}{{\text{O}}_{\text{3}}}{\text{)}}\) high temperature in internal combustion/jet engine;
reaction between \({{\text{N}}_{\text{2}}}\) and \({{\text{O}}_{\text{2}}}\) at high temperature/lightning;
Accept either of the above for first mark.
\({\text{2N}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \to {\text{HN}}{{\text{O}}_3} + {\text{HN}}{{\text{O}}_2}/{\text{4N}}{{\text{O}}_2} + {{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} \to {\text{4HN}}{{\text{O}}_3}\);
Acid 2:
\({\text{(}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{3}}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{)}}\) from burning of coal / smelting plants / sulfuric acid plants / volcanic activity;
Do not accept combustion of fossil fuels.
\({\text{S}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_3}/{\text{S}}{{\text{O}}_3} + {{\text{H}}_2}{\text{O}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_4}\);
Allow H2SO3/H2SO4 to be Acid 1 and HNO2/HNO3 to be Acid 2.
\({\text{CaC}}{{\text{O}}_{\text{3}}} + {\text{2HN}}{{\text{O}}_{\text{3}}} \to {\text{Ca(N}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}} + {\text{C}}{{\text{O}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\);
Accept equation with H2SO3 or H2SO4 or ionic equations.
Do not accept equations with H2CO3.
Examiners report
Most candidates correctly identified carbon dioxide as the source of natural water acidity and wrote an acceptable equation. Many also identified sources of nitric and sulphuric acid, though these equations often proved trickier, with many candidates writing equations for the formation of the oxide from which the acid is derived. Balanced equations for the reaction with limestone also proved to be a challenge, with carbonic acid often appearing as a product.
Most candidates correctly identified carbon dioxide as the source of natural water acidity and wrote an acceptable equation. Many also identified sources of nitric and sulphuric acid, though these equations often proved trickier, with many candidates writing equations for the formation of the oxide from which the acid is derived. Balanced equations for the reaction with limestone also proved to be a challenge, with carbonic acid often appearing as a product.
Most candidates correctly identified carbon dioxide as the source of natural water acidity and wrote an acceptable equation. Many also identified sources of nitric and sulphuric acid, though these equations often proved trickier, with many candidates writing equations for the formation of the oxide from which the acid is derived. Balanced equations for the reaction with limestone also proved to be a challenge, with carbonic acid often appearing as a product.
Question
Nitrogen dioxide and sulfur dioxide are two air pollutants.
Nitrogen dioxide is formed in a two-stage process. Describe one anthropogenic (man-made) source of nitrogen dioxide and state the two chemical equations for its formation.
Both of these air pollutants also contribute to acid deposition. State one chemical equation for each gas to describe how each forms an acidic solution.
▶️Answer/Explanation
Markscheme
combustion of fuels (at high temperature);
Accept internal combustion/aircraft/jet engines.
\({{\text{N}}_2} + {{\text{O}}_2} \to {\text{2NO}}\) and \(2{\text{NO}} + {{\text{O}}_2} \to {\text{2N}}{{\text{O}}_2}\);
\({\text{2N}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \to {\text{HN}}{{\text{O}}_2} + {\text{HN}}{{\text{O}}_3}/{\text{4N}}{{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} + {{\text{O}}_2} \to {\text{4HN}}{{\text{O}}_3}\);
\({\text{S}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_3}/{\text{2S}}{{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} + {{\text{O}}_2} \to {\text{2}}{{\text{H}}_2}{\text{S}}{{\text{O}}_4}\);
Examiners report
The man-made source of nitrogen oxide was generally very well answered, although the equations for its formation proved demanding.
The chemical equation for the formation of sulfuric acid was given correctly by many candidates, but it was surprising to see that a significant number of candidates did not know the chemical formula for nitric acid.
Question
One major environmental problem that affects many countries is acid rain.
Nitrogen monoxide pollution is a major contributor of acid rain.
Explain, writing an appropriate equation, why, even in an unpolluted environment, rainwater is still slightly acidic.
(i) Outline the major source of this gas, including an equation.
(ii) Describe, including an equation, a chemical method used to control the emission of this pollutant.
(iii) Identify a compound, to which nitrogen monoxide is eventually converted, that is responsible for acidity in lakes and rivers.
▶️Answer/Explanation
Markscheme
dissolved carbon dioxide / \({\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(1)}} \rightleftharpoons {{\text{H}}_2}{\text{C}}{{\text{O}}_3}{\text{(aq)}}\);
\({{\text{H}}_2}{\text{C}}{{\text{O}}_3}{\text{(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{HCO}}_3^ – {\text{(aq)}}/{\text{C}}{{\text{O}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(1)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{HCO}}_3^ – {\text{(aq)}}\);
(i) internal combustion engine / high temperature combustion;
\({{\text{N}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2NO(g)}}\);
(ii) catalytic converters / exhaust recirculation;
\(2{\text{NO(g)}} + {\text{2CO(g)}} \to {{\text{N}}_2}{\text{(g)}} + {\text{2C}}{{\text{O}}_2}{\text{(g)}}\);
(iii) nitric acid/\({\text{HN}}{{\text{O}}_{\text{3}}}\) / nitrous acid/\({\text{HN}}{{\text{O}}_{\text{2}}}\);
Examiners report
Many candidates identified that dissolved carbon dioxide causes the rain water to be acidic but did not show with the help of equation partial dissociation of carbonic acid.
The source of nitrogen monoxide and the method used to control its emission appeared to be well known and many could include appropriate chemical equations. Very few candidates could identify that nitric or nitrous acid is responsible for acidity in lakes and rivers. Some candidates formed \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\), \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{3}}}\), \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) and \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) from nitrogen monoxide.
Question
Acid deposition is a major environmental concern. Although it is usually associated with human activities, natural sources can also contribute to this phenomenon.
State one natural origin of acid deposition.
State equations which represent chemical transformations of elemental sulfur into sulfurous acid, H2SO3.
Discuss the possible ways of decreasing acid deposition and its adverse effects on the environment.
▶️Answer/Explanation
Markscheme
volcano eruption/activity / lightning / microbial activity;
\({\text{S}} + {{\text{O}}_{\text{2}}} \to {\text{S}}{{\text{O}}_{\text{2}}}\);
\({\text{S}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_3}\);
washing of coal/natural gas / fluidized bed combustion / remove sulfur before/during the burning;
scrubbing exhaust gases;
using catalytic converters;
low-S diesel / fuel switching / use of alternative energy sources/wind/solar/tidal energy;
reduction in energy consumption;
Accept specific examples of energy saving (e.g. use of bicycles instead of cars).
use lime/formula of a reasonable base on rivers/lakes/soils to neutralize the acid;
Allow other methods/solutions.
Award [2 max] for adverse effects of acid deposition on environment.
Examiners report
This was generally answered well although in (a) some thought the natural acidity of rain, caused by dissolved CO2, was what was required.
Part (b) was answered well (although the number of candidates who wrote elemental sulfur as a diatomic molecule was worrying).
There were some good discussions in (c). Many candidates mis-interpreted the question as asking about the effects of acid rain – credit was given.
Question
Nitrogen monoxide gas, NO, is emitted by cars and leads to acid deposition.
Discuss the damage to the environment caused by acid deposition.
▶️Answer/Explanation
Markscheme
leaches/removes nutrients from soil;
Accept specific ions for nutrients.
plant leaves are damaged;
Do not allow just damages plants.
increasing aluminium concentration in the soil;
root damage;
limestone buildings/rocks/statues react with acid;
lakes become acidic killing fish;
toxic metal ions leached/enter into water supplies;
Examiners report
Most candidates gave specific detail in their answer to the effects of acid deposition gaining partial marks, and about a third of the candidates gained full marks on part (a).
Question
Acid deposition can have a significant impact on aquatic environments such as lakes or wetlands.
State what is meant by the term acid deposition.
Identify one oxide which causes acid deposit
One effect of acid deposition is to decrease the pH of lake water. Suggest how this effect could be reversed.
▶️Answer/Explanation
Markscheme
acidic/acid-forming pollutants deposited on the Earth’s surface/leave the
atmosphere / rain/precipitation/deposition that is acidic/with a \({\text{pH}} < 5.6\);
Award mark if two specific examples are given.
\({\text{S}}{{\text{O}}_{\text{2}}}{\text{/S}}{{\text{O}}_{\text{3}}}{\text{/N}}{{\text{O}}_{\text{2}}}\);
Allow names of oxides. Do not allow NOx.
Accept NO, but for second mark 2NO + O2 \( \to \) 2NO2 must also be included.
\({\text{S}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_3}/{\text{S}}{{\text{O}}_3} + {{\text{H}}_2}{\text{O}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_4}/\)
\({\text{2S}}{{\text{O}}_2} + {{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} \to {\text{2}}{{\text{H}}_2}{\text{S}}{{\text{O}}_4}/{\text{2N}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \to {\text{HN}}{{\text{O}}_2} + {\text{HN}}{{\text{O}}_3}/\)
\({\text{4N}}{{\text{O}}_2} + {{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} \to {\text{4HN}}{{\text{O}}_3}\);
Do not allow ECF for equation.
addition of lime/Ca(OH)2/limestone/CaCO3;
Accept “adding alkali/base” or “neutralizing acidity”.
Examiners report
Though the precise definition of “acid deposition” was rarely encountered, most candidates managed a reply that gained them the mark. Even the simple equations required for the reaction of the oxide with water proved difficult for many candidates and, even though most knew how to counteract lake acidity, a disappointing number of students failed to link their method of reducing emissions to the oxide selected, for example mentioning catalytic converters in reference to oxides of sulfur.
Though the precise definition of “acid deposition” was rarely encountered, most candidates managed a reply that gained them the mark. Even the simple equations required for the reaction of the oxide with water proved difficult for many candidates and, even though most knew how to counteract lake acidity, a disappointing number of students failed to link their method of reducing emissions to the oxide selected, for example mentioning catalytic converters in reference to oxides of sulfur.
Though the precise definition of “acid deposition” was rarely encountered, most candidates managed a reply that gained them the mark. Even the simple equations required for the reaction of the oxide with water proved difficult for many candidates and, even though most knew how to counteract lake acidity, a disappointing number of students failed to link their method of reducing emissions to the oxide selected, for example mentioning catalytic converters in reference to oxides of sulfur.
Question
The normal pH of rainwater is 5.6, but in some parts of the world rainwater has been recorded with a pH of several units lower than this. This is associated with harmful effects on living and non-living things.
The decrease in the pH of rainwater is mainly caused by oxides of non-metals, principally nitrogen and sulfur. State chemical equations that show how the primary pollutant nitrogen(II) oxide can produce two different acids containing nitrogen.
Explain, including an equation, the effect of the acid rain produced in (a) on certain stone buildings.
▶️Answer/Explanation
Markscheme
\({\text{2NO(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2N}}{{\text{O}}_{\text{2}}}{\text{(g)}}\);
\({\text{2N}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{HN}}{{\text{O}}_2}{\text{(aq)}} + {\text{HN}}{{\text{O}}_3}{\text{(aq)}}\);
Ignore state symbols.
Award [1 max] for 4NO2(aq) + 2H2O(l) + O2(g) \( \to \) 4HNO3(aq).
erosion / buildings of marble/limestone;
\({\text{2HN}}{{\text{O}}_3}{\text{(aq)}} + {\text{CaC}}{{\text{O}}_3}{\text{(s)}} \to {\text{Ca(N}}{{\text{O}}_3}{{\text{)}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\) /
\({\text{2HN}}{{\text{O}}_2}{\text{(aq)}} + {\text{CaC}}{{\text{O}}_3}{\text{(s)}} \to {\text{Ca(N}}{{\text{O}}_2}{{\text{)}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\);
Ignore state symbols.
Examiners report
This question was not done well. Few candidates were able to form the two different nitrogen containing acids and the acid used in (b) was generally sulfuric!
This question was not done well. Few candidates were able to form the two different nitrogen containing acids and the acid used in (b) was generally sulfuric!
Question
Acid deposition is a consequence of industrial processes.
State what is meant by the term acid deposition.
Industrial processes, such as the burning of coal, generate non-metallic oxides of carbon and nitrogen into the atmosphere. State balanced equations for the reactions by which these oxides are produced and then removed from the atmosphere.
Oxide of carbon:
Produced:
Removed:
Oxide of nitrogen:
Produced:
Removed:
All shellfish have a calcium carbonate shell. Discuss, including a balanced equation, the long-term effect of acid deposition on these organisms.
Balanced equation:
▶️Answer/Explanation
Markscheme
process by which acidic (substances) leave atmosphere/return to Earth / OWTTE;
Do not allow acid rain.
Oxide of carbon:
Produced: \({\text{C(s)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}}\);
Accept a correctly balanced equation for the combustion of a hydrocarbon fuel.
Removed: \({\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{C}}{{\text{O}}_3}{\text{(aq)}}/{\text{6C}}{{\text{O}}_2}{\text{(g)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_6}{\text{(aq)}} + {\text{6}}{{\text{O}}_2}{\text{(g)}}\);
OR
Produced: \({\text{2C(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2CO(g)}}\);
Removed: \({\text{2CO(g)}} + {\text{2NO(g)}} \to {{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{2C}}{{\text{O}}_2}{\text{(g)}}/{\text{2CO(g)}} + {{\text{O}}_2} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}}\);
Oxide of nitrogen:
Produced: \({{\text{N}}_2}{\text{(g)}} + {\text{2}}{{\text{O}}_2}{\text{(g)}} \to {\text{2N}}{{\text{O}}_2}{\text{(g)}}/{\text{2NO(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2N}}{{\text{O}}_2}{\text{(g)}}\);
Removed: \({\text{2N}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{HN}}{{\text{O}}_3}{\text{(aq)}} + {\text{HN}}{{\text{O}}_2}{\text{(aq)}}\) /
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{4N}}{{\text{O}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{4HN}}{{\text{O}}_3}{\text{(aq)}}\);
OR
Produced: \({{\text{N}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2NO(g)}}\);
Removed: \({\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{4NO(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{4HN}}{{\text{O}}_2}{\text{(aq)}}/{\text{2NO(g)}} + {{\text{O}}_2} \to {\text{2N}}{{\text{O}}_2}{\text{(g)}}\) /
\({\text{2CO(g)}} + {\text{2NO(g)}} \to {{\text{N}}_2}{\text{(g)}} + {\text{2C}}{{\text{O}}_2}{\text{(g)}}\);
Ignore state symbols.
shells become thinner as some of the calcium carbonate shell reacts / OWTTE;
Accept “dissolving of marine carbonate shells”.
\({\text{CaC}}{{\text{O}}_3}{\text{(s)}} + {\text{2HN}}{{\text{O}}_3}{\text{(aq)}} \to {\text{Ca(N}}{{\text{O}}_3}{{\text{)}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\) /
\({\text{CO}}_3^{2 – }{\text{(s)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\) /
\({\text{CaC}}{{\text{O}}_3}{\text{(s)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{a}}^{2 + }}{\text{(aq)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\) /
\({\text{CaC}}{{\text{O}}_3}{\text{(s)}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} \to {\text{CaS}}{{\text{O}}_4}{\text{(aq)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
Allow equations with H2SO3 and HNO2.
Do not accept H2CO3 instead of H2O and CO2.
Examiners report
Many correct answers. Some candidates oversimplified the term as acid rain.
While this question received many correct answers it is worrying that a number of candidates used incorrect formulae for common substances (such as N instead of \({{\text{N}}_{\text{2}}}\)) and a few did not balance their equations.
Most candidates realized that the acid would react with the carbonate rendering the shell weaker and therefore obtained at least one mark. The second mark was scored by a smaller number of candidates. Some candidates lost the second mark by using an acid not found in acid deposition or using the wrong formula for the salt product.

