Question
Which causes acid deposition?
A S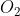
B Si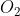
C SrO
D C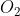
▶️Answer/Explanation
Ans: A
Acid deposition forms when sulfur dioxide (SO2) and nitrogen oxides (NOx) combine with moisture in the atmosphere to produce sulfuric acid and nitric acid.
Question
Which of these oxides contribute to acid deposition?
(i) SO2
(ii) NO2
(iii) CO2
A I and II only
B I and III only
C II and III only
D I, II and III
▶️Answer/Explanation
Ans: A
Acid deposition forms when sulfur dioxide (SO2) and nitrogen oxides (NOx) combine with moisture in the atmosphere to produce sulfuric acid and nitric acid.
Question
Normal rain is?
A Slightly acidic
B Slightly basic
C Neutral
D Strongly acidic
▶️Answer/Explanation
Ans: A
Normal rain is slightly acidic with pH about 5.6 due to carbon dioxide dissolving into it forming weak carbonic acid.
Question
Which of the following is present in maximum amount in acid deposition?
A Sulfuric acid
B Hydrochloric acid
C Carbonic acid
D Carboxylic acid
▶️Answer/Explanation
Ans: A
Acid deposition forms when sulfur dioxide (SO2) and nitrogen oxides (NOx) combine with moisture in the atmosphere to produce sulfuric acid and nitric acid.
Sulfuric acid is present in maximum amount.
